- Stock: In Stock
- Package: 5mg Vial + 1ml water
Ask a Question About This Product
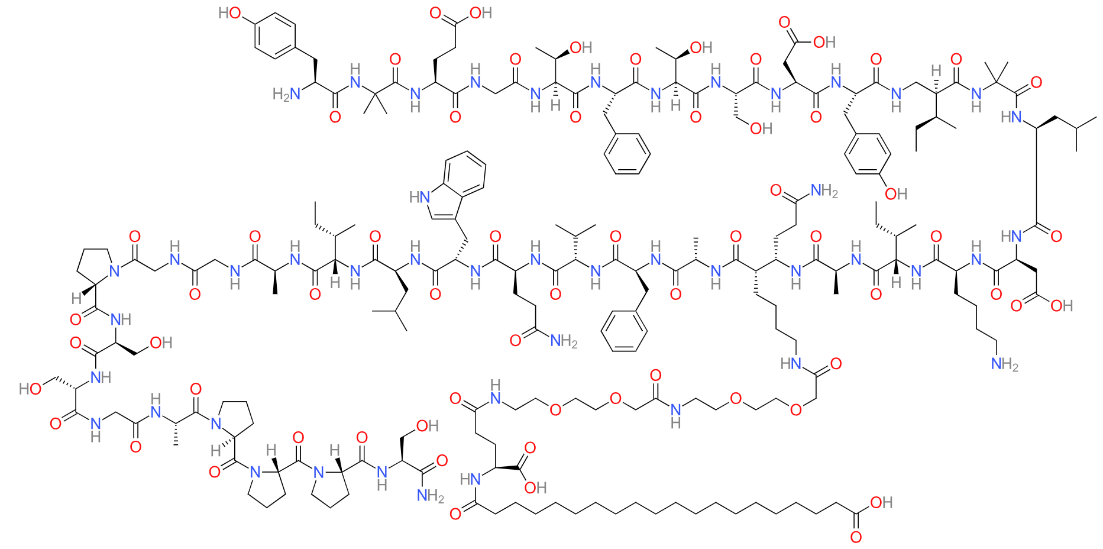
Tirzepatide is an innovative pharmacological agent that represents a significant advancement in the treatment of type 2 diabetes mellitus (T2DM) and obesity. Developed by Eli Lilly, this drug is a dual agonist that targets both the glucose-dependent insulinotropic polypeptide (GIP) receptor and the glucagon-like peptide-1 (GLP-1) receptor. These incretin hormones are naturally occurring peptides involved in the regulation of glucose homeostasis and energy balance. By simultaneously targeting these pathways, tirzepatide exerts a synergistic effect that enhances its efficacy in managing hyperglycemia and promoting weight loss, distinguishing it from existing therapies that typically target only the GLP-1 receptor.
The development of tirzepatide is rooted in the growing recognition of the need for more effective therapeutic options for patients with T2DM, particularly those who are also struggling with obesity. Traditional antidiabetic medications often fall short in addressing the multifaceted needs of these patients, especially in terms of weight management, which is a critical component of comprehensive diabetes care. Tirzepatide’s dual mechanism not only addresses the glucose control but also significantly impacts body weight, an area where many other therapies have limited success. The drug’s ability to achieve such significant outcomes has led to its consideration as a potential game-changer in both endocrinology and metabolic disease management.
Recent clinical trials have highlighted tirzepatide's remarkable efficacy. For instance, the SURPASS program, a series of Phase III clinical trials, demonstrated that tirzepatide could achieve superior reductions in HbA1c levels and body weight compared to other established therapies, including insulin and GLP-1 receptor agonists like semaglutide. The dual incretin agonism of tirzepatide offers a more comprehensive approach to T2DM and obesity, making it particularly appealing for patients who require both glycemic control and substantial weight loss. Furthermore, the drug's effects extend beyond these primary outcomes, potentially offering benefits in cardiovascular health and overall metabolic profile, making it an attractive option not just for diabetologists, but also for specialists in cardiology and endocrinology.
Given the growing epidemic of obesity and diabetes worldwide, the introduction of tirzepatide is timely. Its potential to address the dual challenges of hyperglycemia and obesity could redefine the standard of care for millions of patients globally. Moreover, its off-label use is already being explored in the bodybuilding and fitness communities, where its potent weight loss effects could provide an edge in fat reduction and metabolic optimization. However, the full spectrum of tirzepatide’s potential is still being studied, and ongoing research will likely continue to expand our understanding of its benefits and applications.
Key Effects and Benefits
Weight Loss
Tirzepatide has been shown to produce unprecedented levels of weight loss in clinical trials, which is particularly noteworthy given the historical difficulty of achieving significant weight reduction in patients with T2DM or obesity. In the SURMOUNT-1 trial, a Phase III study focused on weight loss, participants treated with the highest dose of tirzepatide (15 mg) achieved an average weight loss of 22.5% over 72 weeks. This is a substantial improvement over the weight loss achieved with other anti-obesity medications, including semaglutide, which typically results in weight reductions of approximately 15-17%. The weight loss effect of tirzepatide is attributed to its dual action on GIP and GLP-1 receptors, which together enhance satiety, reduce food intake, and increase energy expenditure.
For bodybuilders and fitness enthusiasts, this weight loss effect is of particular interest, as it may help in achieving a leaner physique with reduced body fat while preserving muscle mass. This is crucial in contexts where maintaining muscle definition and overall body composition is paramount. Furthermore, the reduction in visceral fat, which is often resistant to diet and exercise alone, is another significant benefit, as visceral fat is associated with increased cardiometabolic risk.
Glycemic Control
Tirzepatide's efficacy in lowering blood glucose levels is another major benefit. In clinical trials, the drug has consistently demonstrated superior HbA1c reductions compared to other treatments. For example, in the SURPASS-2 trial, tirzepatide at the 15 mg dose reduced HbA1c by 2.58% from baseline, outperforming semaglutide, which achieved a reduction of 2.07%. This superior glycemic control is vital for patients with T2DM, as maintaining lower HbA1c levels reduces the risk of complications such as neuropathy, nephropathy, and retinopathy.
For athletes and bodybuilders, maintaining stable blood glucose levels is critical for optimizing performance, recovery, and overall metabolic health. Tirzepatide’s ability to provide robust glycemic control without significantly increasing the risk of hypoglycemia (thanks to its glucose-dependent mechanism) makes it a particularly attractive option for those who require stringent management of their blood sugar levels.
Cardiovascular Benefits
Beyond its effects on weight and glucose levels, tirzepatide may also offer cardiovascular benefits. The drug has been shown to reduce several cardiovascular risk factors, including body weight, systolic blood pressure, and lipid levels. These effects are particularly relevant for patients with T2DM, who are at an elevated risk for cardiovascular disease. Although long-term cardiovascular outcomes trials are still ongoing, the initial data suggest that tirzepatide could play a role in reducing cardiovascular events, such as heart attacks and strokes, in high-risk populations.
Mechanism of Action
Tirzepatide’s mechanism of action is unique due to its dual agonism of both the GIP and GLP-1 receptors, which are integral to glucose metabolism and energy homeostasis.
GIP Receptor Agonism
GIP is an incretin hormone released by the K-cells of the small intestine in response to nutrient ingestion, particularly fats and carbohydrates. Its primary function is to enhance insulin secretion from the pancreatic beta cells in a glucose-dependent manner. However, GIP also plays a role in lipid metabolism and adipogenesis. By agonizing the GIP receptor, tirzepatide enhances insulin secretion in response to meals, which helps to control postprandial blood glucose levels. This insulinotropic effect is critical for managing T2DM, as it reduces hyperglycemia without significantly increasing the risk of hypoglycemia, a common concern with other insulinotropic agents.
Interestingly, GIP receptor agonism also influences fat metabolism. In preclinical studies, GIP has been shown to promote lipid storage in adipose tissue, but in the context of tirzepatide’s dual action, this effect may be counterbalanced by GLP-1 receptor activation, which promotes weight loss. The net effect is a reduction in body fat, particularly visceral fat, which is associated with improved metabolic health.
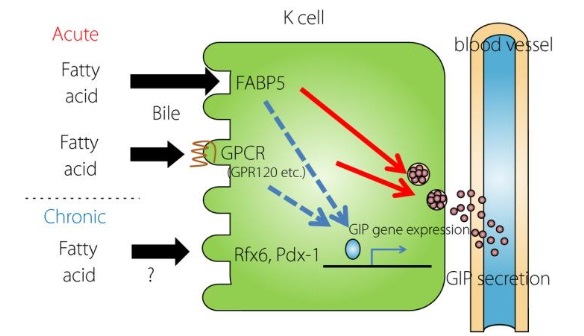
The process of GIP (gastric inhibitory polypeptide/glucose-dependent insulinotropic polypeptide) secretion and its enhanced synthesis in response to a high-fat diet (HFD) involves several key mechanisms. Fatty acid-binding protein 5 (FABP5) and G protein-coupled receptor 120 (GPR120) are crucial for the immediate secretion of GIP following fat intake. Additionally, in the context of obesity induced by HFD, the transcription factor regulatory factor X6 (Rfx6) plays a significant role in the hypersecretion of GIP by upregulating the expression of the GIP gene. These processes are interconnected with other components such as GPCR (G protein-coupled receptors) and Pdx-1 (pancreatic and duodenal homeobox 1).
GLP-1 Receptor Agonism
GLP-1, another incretin hormone, is secreted by the L-cells of the intestine in response to nutrient intake. It enhances insulin secretion, suppresses glucagon release, slows gastric emptying, and promotes satiety. GLP-1 receptor agonists have become a cornerstone in the treatment of T2DM due to their ability to lower blood glucose levels while also promoting weight loss. Tirzepatide’s action on the GLP-1 receptor contributes significantly to its effects on satiety and weight reduction. By slowing gastric emptying, tirzepatide prolongs the feeling of fullness after meals, reducing overall caloric intake. Additionally, the suppression of glucagon, a hormone that raises blood glucose levels, further aids in maintaining glycemic control.
The dual activation of GIP and GLP-1 receptors by tirzepatide results in a synergistic effect that enhances its therapeutic potential. While GLP-1 receptor agonism is primarily responsible for the weight loss and appetite suppression observed with tirzepatide, GIP receptor agonism complements these effects by further enhancing insulin secretion and influencing lipid metabolism. This combination allows tirzepatide to address both hyperglycemia and obesity more effectively than agents targeting only one of these pathways.
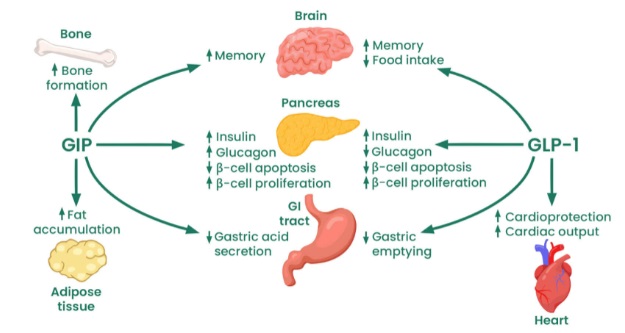
Pancreatic and exopancreatic function of glucose‐dependent insulinotropic polypepide (GIP) and glucagon‐like peptide (GLP)‐1. GIP acts directly on the endocrine pancreas, bone, fat, gastrointestinal (GI) tract and brain. GLP‐1 acts directly on the endocrine pancreas, gastrointestinal tract, heart and brain.
Beta-Cell Function Enhancement
Beyond enhancing insulin secretion, tirzepatide may also play a crucial role in preserving and enhancing pancreatic beta-cell function. Chronic activation of GIP and GLP-1 receptors has been shown to promote beta-cell proliferation and reduce apoptosis, which is the programmed cell death that often contributes to the decline in beta-cell mass observed in T2DM. By supporting beta-cell survival and potentially increasing their number, tirzepatide may help maintain a healthier beta-cell population over time. This preservation is crucial for maintaining long-term glycemic control in patients with T2DM and could potentially delay the progression of the disease, reducing the need for exogenous insulin and mitigating the risk of diabetes-related complications.
Impact on Hepatic Glucose Production
Tirzepatide’s ability to suppress glucagon secretion has significant implications for hepatic glucose production, a key factor in managing fasting blood glucose levels. By reducing glucagon levels, tirzepatide decreases gluconeogenesis, the process by which the liver produces glucose from non-carbohydrate sources, and glycogenolysis, the breakdown of glycogen into glucose. Both processes are major contributors to fasting hyperglycemia in diabetes. This reduction in hepatic glucose output, combined with the enhancement of peripheral glucose uptake through increased insulin sensitivity, results in more effective overall glucose control, particularly in reducing fasting glucose levels and smoothing out daily glucose fluctuations.
Modulation of Lipid Metabolism
In addition to its effects on glucose metabolism, tirzepatide plays a significant role in lipid metabolism, particularly in how the body handles dietary fats. By influencing the GIP pathway, tirzepatide may reduce the accumulation of triglycerides in adipose tissue, which is crucial as excess triglycerides, especially in visceral fat, are linked to insulin resistance and increased cardiovascular risk. Furthermore, tirzepatide might enhance fat oxidation, the process by which fats are broken down to be used as energy, contributing to weight loss and improved lipid profiles. These effects include reductions in LDL cholesterol and increases in HDL cholesterol, further supporting the weight loss and cardiovascular benefits associated with tirzepatide therapy. For patients, these metabolic improvements can lead to reduced visceral fat, which is associated with better insulin sensitivity and a lower risk of metabolic syndrome, a cluster of conditions that increases the risk of heart disease, stroke, and diabetes.
Anti-Inflammatory Effects
Recent studies suggest that tirzepatide may also have anti-inflammatory properties, which could be mediated through GLP-1 receptor activation. Chronic low-grade inflammation is a hallmark of metabolic diseases such as T2DM and obesity and plays a significant role in the development of insulin resistance and cardiovascular complications. By reducing levels of inflammatory cytokines, such as C-reactive protein (CRP) and interleukins, tirzepatide may help to mitigate this inflammation, thereby improving insulin sensitivity and reducing cardiovascular risk. Additionally, by improving endothelial function, which is often impaired in patients with diabetes and obesity, tirzepatide could further enhance cardiovascular health. This reduction in inflammation and improvement in endothelial function offer additional protective benefits against cardiovascular disease, further enhancing the therapeutic profile of tirzepatide.
Improvement in Cardiovascular Health
Tirzepatide's effects on cardiovascular health extend beyond its metabolic actions. The activation of GLP-1 receptors in vascular endothelial cells improves endothelial function, which is crucial for maintaining healthy blood vessels and reducing arterial stiffness, a common issue in patients with T2DM that contributes to hypertension and cardiovascular disease. By improving the elasticity of arteries and reducing blood pressure, tirzepatide may reduce the risk of developing cardiovascular complications such as stroke and heart attack. Coupled with its effects on weight loss and improved glycemic control, tirzepatide presents a comprehensive treatment option for managing not only diabetes but also its associated cardiovascular risks, making it a highly versatile and effective therapeutic agent in the fight against metabolic diseases.
Comparison Between Tirzepatide and Semaglutide
Tirzepatide and semaglutide are both incretin-based therapies, but they differ significantly in their mechanisms of action, efficacy, and potential uses.
Mechanism of Action
Semaglutide is a GLP-1 receptor agonist that mimics the action of the endogenous hormone GLP-1, enhancing insulin secretion, suppressing glucagon, slowing gastric emptying, and promoting satiety. Tirzepatide, on the other hand, is a dual GIP and GLP-1 receptor agonist, offering a broader mechanism of action that includes the effects of both incretin hormones. This dual agonism allows tirzepatide to have a more pronounced effect on both glucose metabolism and weight reduction compared to semaglutide.
Efficacy
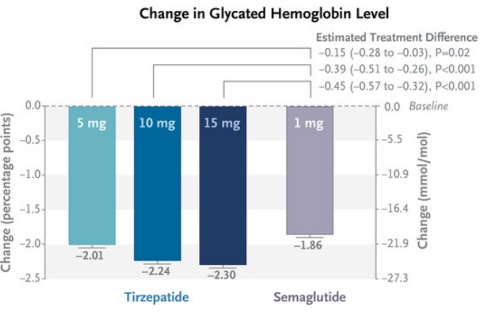
The efficacy of tirzepatide compared to semaglutide in patients with type 2 diabetes was evaluated by measuring the reduction in glycated hemoglobin (HbA1c) levels and body weight after 40 weeks of treatment. The study utilized three different doses of tirzepatide (5 mg, 10 mg, and 15 mg) and compared these to a standard 1 mg dose of semaglutide (doi: 10.1056/NEJMoa2107519).
Reduction in Glycated Hemoglobin (HbA1c):
- All doses of tirzepatide showed a greater reduction in HbA1c levels compared to semaglutide.
- The 5 mg dose of tirzepatide resulted in a mean HbA1c reduction of approximately 2.01%, which is greater than the 1.86% reduction observed with semaglutide.
- Higher doses of tirzepatide (10 mg and 15 mg) provided even more significant reductions, with decreases of 2.24% and 2.30%, respectively.
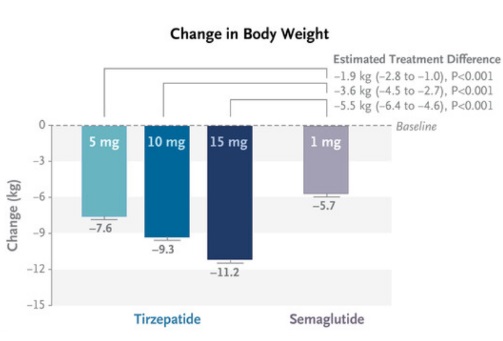
Reduction in Body Weight:
- Tirzepatide also demonstrated superior efficacy in weight loss compared to semaglutide across all doses.
- Patients receiving the 5 mg dose of tirzepatide lost an average of 7.6 kg, which is more than the 5.7 kg lost with semaglutide.
- The 10 mg and 15 mg doses of tirzepatide led to even greater weight reductions, with mean losses of 9.3 kg and 11.2 kg, respectively.
- Overall, tirzepatide was more effective than semaglutide in both reducing glycated hemoglobin levels and promoting weight loss in patients with type 2 diabetes.
Applications
While both drugs are used for the management of T2DM, tirzepatide’s additional effects on weight loss make it a more versatile option for patients with obesity. Semaglutide is also approved for chronic weight management under the brand name Wegovy, but tirzepatide's superior weight loss efficacy may make it a preferred choice in this context once it receives approval for this indication. Additionally, tirzepatide’s impact on lipid metabolism and potential cardiovascular benefits may offer further advantages in treating patients with metabolic syndrome or those at high cardiovascular risk.
Side Effects
Both drugs have similar side effect profiles, with gastrointestinal symptoms being the most common. However, tirzepatide’s dual mechanism may lead to slightly different tolerability profiles, which will require further study to fully understand. Long-term safety data are more established for semaglutide, given its longer time on the market, but early indications suggest that tirzepatide’s safety profile is comparable.
Uses
Tirzepatide is used for several key purposes, each with distinct goals and outcomes.
Type 2 Diabetes Management
Tirzepatide is primarily indicated for the treatment of T2DM to achieve improved glycemic control. The drug’s dual mechanism allows it to reduce HbA1c levels more effectively than many existing treatments, making it an ideal choice for patients who struggle to reach their glycemic targets with other medications. The combination of GIP and GLP-1 receptor agonism addresses both fasting and postprandial hyperglycemia, reducing the risk of long-term complications associated with poor glycemic control, such as cardiovascular disease, neuropathy, and retinopathy.
The goal of using tirzepatide in T2DM is not only to lower HbA1c levels but also to improve overall metabolic health. By reducing body weight, lowering blood pressure, and improving lipid profiles, tirzepatide helps mitigate the broader metabolic risks associated with diabetes. For patients with obesity and T2DM, tirzepatide offers the dual benefit of glycemic control and significant weight loss, which can further enhance insulin sensitivity and reduce the need for additional medications.
Weight Management and Obesity
Tirzepatide is being explored for its potential in treating obesity, even in patients without diabetes. The drug’s ability to induce significant weight loss makes it a promising candidate for addressing the obesity epidemic, which is a major risk factor for numerous chronic diseases, including cardiovascular disease, certain cancers, and osteoarthritis. The goal of using tirzepatide in this context is to achieve sustainable weight loss that improves overall health outcomes, reduces comorbidities, and enhances quality of life.
In the context of obesity management, tirzepatide is particularly useful for patients who have not achieved sufficient weight loss with lifestyle interventions alone or who have struggled with weight regain after initial success. The drug’s ability to reduce hunger and increase satiety supports adherence to a reduced-calorie diet, which is essential for long-term weight management. Moreover, the weight loss achieved with tirzepatide is accompanied by improvements in metabolic parameters, such as reduced waist circumference, improved insulin sensitivity, and lower levels of inflammatory markers, all of which contribute to better health outcomes (https://doi.org/10.1038/s41591-023-02597-w)
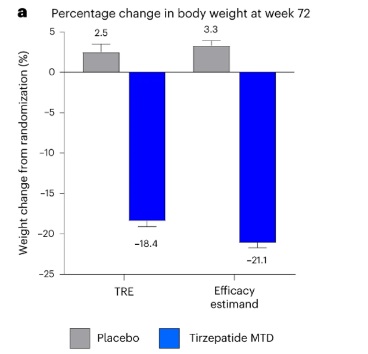
The chart shows that Tirzepatide significantly reduces body weight by 18.4% to 21.1% at week 72 compared to a slight weight gain of 2.5% to 3.3% with the placebo. This highlights Tirzepatide as an effective treatment for substantial weight loss.
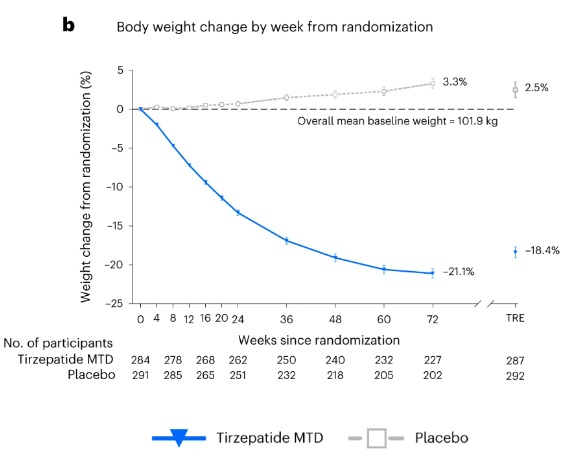
The graph illustrates that participants taking Tirzepatide experienced a significant and steady weight reduction, reaching a 21.1% decrease by week 72. In contrast, those on the placebo saw a slight weight gain of 3.3% over the same period. The baseline mean body weight of participants was 101.9 kg, highlighting the substantial impact ofTirzepatide on weight loss.
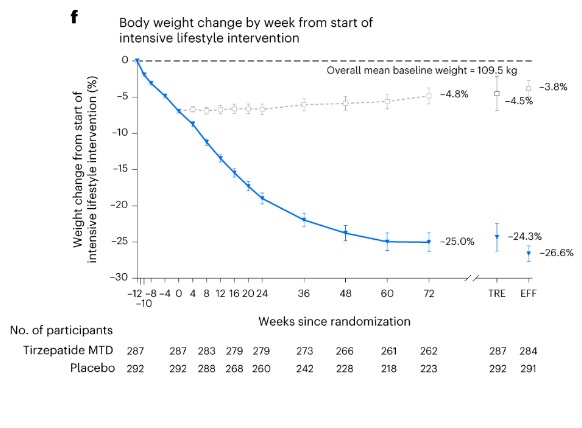
The graph shows the percentage change in body weight from the start of an intensive lifestyle intervention over a 72-week period, comparing Tirzepatide with a placebo. The Tirzepatide group experienced a significant reduction in body weight, with an average decrease of 25.0% at 72 weeks, compared to a 4.8% decrease in the placebo group. The results suggest that Tirzepatide is more effective in achieving weight loss compared to a placebo over the long term.
Off-Label Use in Bodybuilding and Fitness
Although not officially approved for use in bodybuilding or fitness, tirzepatide is gaining attention in these communities for its potent effects on body fat reduction. Bodybuilders often seek to reduce body fat while maintaining or even increasing muscle mass, a goal that tirzepatide may help achieve due to its strong impact on fat loss without significant muscle catabolism. The goal in this context is to enhance body composition, reduce visceral fat, and improve metabolic efficiency, all of which contribute to better athletic performance and a more defined physique.
However, the off-label use of tirzepatide in this context should be approached with caution, as the long-term effects of the drug in individuals without metabolic diseases are not yet fully understood.
Dosage, Administration, and Precautions
Dosage
- Start with a dose of 2.5 mg administered subcutaneously once weekly. This dose is intended to initiate therapy and minimize gastrointestinal side effects.
- After four weeks on the initial dose, the dosage should be increased to 5 mg once weekly.
- If additional glycemic control is required, further increases in dosage can be made in 2.5 mg increments, at intervals of at least four weeks, up to a maximum dose of 15 mg once weekly.
Administration
- Injection Sites: Tirzepatide should be injected subcutaneously in the abdomen, thigh, or upper arm. It is recommended to rotate the injection site with each administration to avoid irritation. The injection should not be administered into areas where the skin is tender, bruised, red, or hard.
- Administration Timing: Tirzepatide can be administered at any time of day, with or without food. The day of the week for the injection can be changed as long as the time between doses is at least three days (72 hours).
- Missed Dose: If a dose is missed, it should be administered as soon as possible within 96 hours (4 days). If more than 96 hours have passed, the missed dose should be skipped, and the next dose should be administered at the regular time.
Precautions
Tirzepatide is an appropriate treatment for most people who need pharmacological support to lose weight. However, there are a few contraindications associated with this weight management medication, including:
- Personal or family history of medullary thyroid carcinoma
- Patients with Multiple Endocrine Neoplasia syndrome type 2
- Known serious hypersensitivity to Tirzepatide
- Women who are pregnant, nursing, or trying to conceive
- Individuals who are taking another GLP-1 agonist, like Semaglutide or Liraglutide
- Individuals with Type 1 diabetes mellitus
- Individuals with a history of pancreatitis. If patients suspect they may have pancreatitis, they should discontinue the medication immediately.
Side Effects
Very Common (10% or more of patients):
- Nausea (up to 18%)
- Diarrhea (up to 17%)
- Decreased appetite (up to 11%)
- Nasopharyngitis (common cold symptoms, up to 10.5%)
- Hypoglycemia (especially when used with insulin or sulfonylureas, up to 19%)
Common (1% to 10% of patients):
- Vomiting
- Constipation
- Abdominal pain
- Dyspepsia (indigestion)
- Eructation (belching)
- Flatulence
- Gastroesophageal reflux disease (GERD)
- Injection site reactions (e.g., pain, redness)
- Fatigue
- Hyperglycemia
- Hypersensitivity reactions (e.g., urticaria, eczema)
Uncommon (0.1% to 1% of patients):
- Cholelithiasis (gallstones)
- Acute pancreatitis
- Increased serum pancreatic enzymes (amylase, lipase)
- Acute gallbladder disease (including biliary colic and cholecystectomy)
Rare and Postmarketing Reports:
- Anaphylaxis
- Angioedema
- Acute kidney injury (especially in patients with pre-existing renal conditions)
- Thyroid tumors (specifically in patients with a history of medullary thyroid carcinoma)
Storage
- Store tirzepatide in the refrigerator at 2°C to 8°C.
- Do not freeze the medication; discard if frozen.
- Keep the medication in its original carton to protect it from light.
- If needed, tirzepatide can be stored at room temperature (up to 30°C) for up to 21 days.
- Do not return the medication to the refrigerator after it has been stored at room temperature.
- Store the prefilled pen in its original packaging until use.
- Dispose of the pen properly after use, following local guidelines.